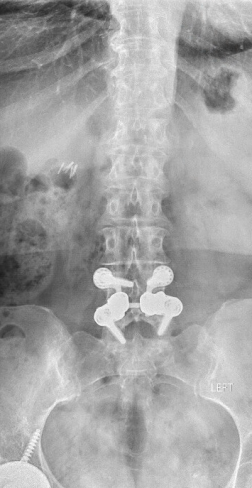
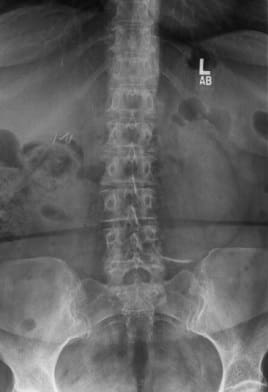
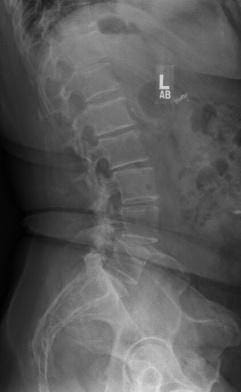
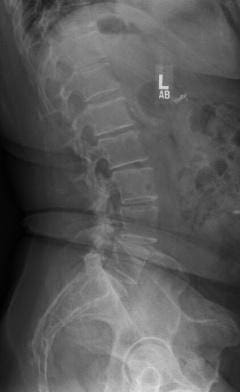
In 2013, this patient from Pismo Beach presented to Dr. Anand looking for a way to continue living her life free of the chronic pain in her lower back that had been interfering with her life for the prior five years. Her images revealed a spondylolisthesis, lumbar stenosis, and facet arthropathy at L4-5. At that time, Dr. Anand was a Principal Investigator for a FDA Investigational Device Exemption study comparing a fusion to an investigational, motion-preserving facet replacement device. Dr. Anand determined that she was an excellent candidate for the study.
“I was very confident I was in the right place after meeting Dr. Anand and his assistant Sheila. They became my family!”
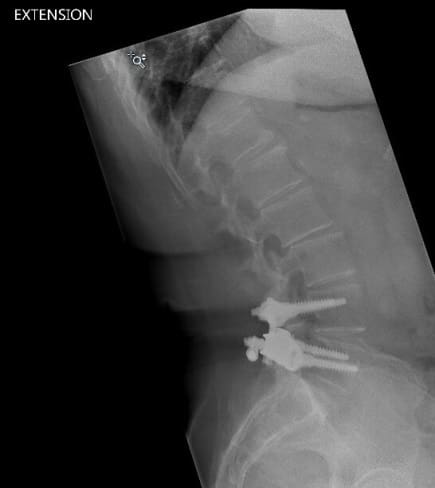
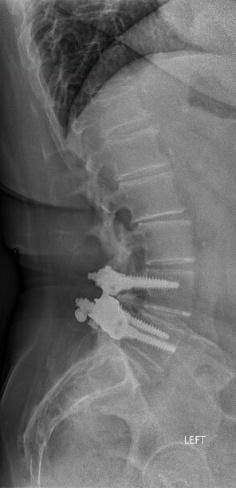
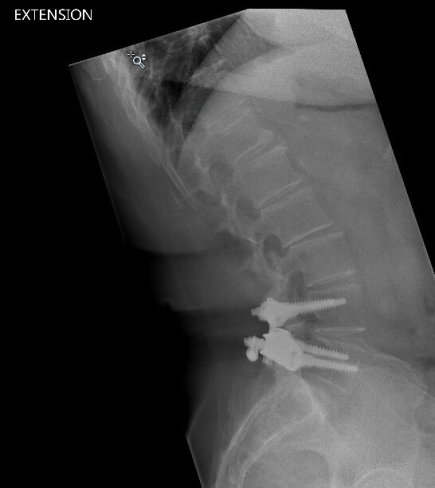
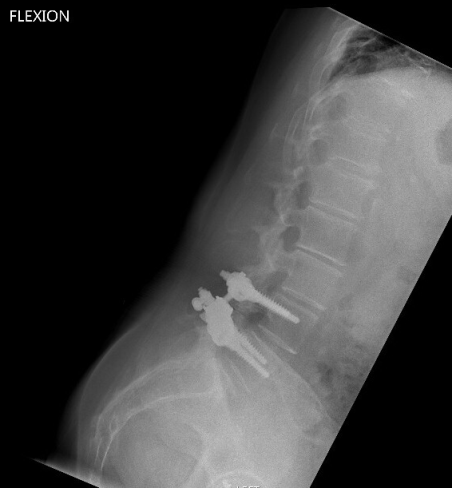
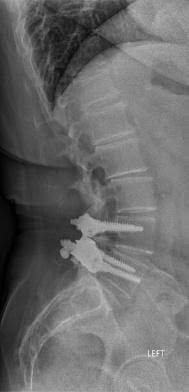
In April 2013, she underwent surgery and was randomized to the experimental arm of the study. She received the facet replacement device at L4-5. In her own words, “When I woke up, the pain, the pain I suffered with for years, was GONE!” She continues to do remarkably well over the past several years and post-operative images demonstrate that the device has successfully maintained motion at the L4-5 segment.
While this particular motion-preservation device is not currently available, Dr. Anand continues his involvement in FDA trials and investigations in novel surgical techniques to treat spinal diseases.